BOUTIQUEResouces
Ustekinumab is a human therapeutic monoclonal antibody targeting p40, the common subunit of interleukin-12 and interleukin-23 (IL-12/23). These two cytokines play a fundamental role in immune-mediated inflammatory diseases such as Crohn’s disease, psoriasis, and multiple sclerosis. The Ustekinumab Drug Concentration Detection Kit is used to measure the concentration of free ustekinumab (e.g., Stelara®), providing attending physicians with the opportunity for early monitoring and treatment optimization.
Immundiagnostik (IDK) GmbH (Germany), founded in 1986, is a global supplier of diagnostic reagents and services. It offers a comprehensive range of in vitro diagnostic (IVD) kits for clinical disease research, covering areas including cardiovascular diseases, musculoskeletal disorders, oxidative stress, and oncology. These kits are applicable for detecting samples from different matrices (e.g., blood, urine, feces, tissue culture supernatants, etc.) and across various species (e.g., humans, mice, rats, etc.).
The IDKmonitor® Ustekinumab Drug Concentration Detection Kit from Immundiagnostik (IDK) is designed for the in vitro quantitative determination of free therapeutic ustekinumab antibodies (e.g., Stelara®) in human serum and EDTA plasma. For Research Use Only.

|
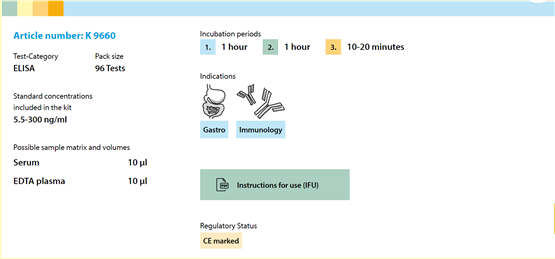
Item Number |
K 9660 |
|
Specification |
96Test |
|
Sample Type |
Serum, EDTA Plasma |
|
Sample size |
10μl |
|
Incubation Time |
1h/1h/10-20min |
|
Storage Conditions |
This product remains stable until the indicated expiration date when stored at 2–8°C. |
|
Applicable Instruments |
It is suitable for all fully automatic and semi-automatic microplate readers with wavelengths of 450 nm and 620 nm. |
More Monoclonal Antibody Drug Concentration Detection:
|
Product Name |
Item Number |
|
IDKmonitor® Free Anti-Infliximab Antibody Detection Kit |
K9650 |
|
IDKmonitor® Total Anti-Infliximab Antibody Detection Kit |
K9654 |
|
IDKmonitor® Infliximab Drug Concentration Detection Kit |
K9655 |
|
IDKmonitor® Free Anti-Adalimumab Antibody Detection Kit |
K9652 |
|
IDKmonitor® Total Anti-Adalimumab Antibody Detection Kit |
K9651 |
|
IDKmonitor® Adalimumab Drug Concentration Detection Kit |
K9657 |
|
IDKmonitor® Free Anti-Etanercept Antibody Detection Kit |
K9653 |
|
IDKmonitor® Etanercept Drug Concentration Detection Kit |
K9646 |
|
IDKmonitor® Free Anti-Golimumab Antibody Detection Kit |
K9649 |
|
IDKmonitor® Golimumab Drug Concentration Detection Kit |
K9656 |
|
IDKmonitor® Free Anti-Vedolizumab Antibody Detection Kit |
K9648 |
|
IDKmonitor® Vedolizumab Drug Concentration Detection Kit |
K9658 |
|
IDKmonitor® Free Anti-Ustekinumab Antibody Detection Kit |
K9666 |
|
IDKmonitor® Total Anti-Ustekinumab Antibody Detection Kit |
K9667 |
|
IDKmonitor® Ustekinumab Drug Concentration Detection Kit |
K9660 |
Beijing XMJ Technology Co., Ltd. is the China distributor of Immundiagnostik (IDK), providing customers with comprehensive technical support and after-sales service. For more information, please feel free to send email to info@xmjsci.com or visit the official website at www.mb020.com.cn.


.png) 京公網(wǎng)安備 11010802028692號
京公網(wǎng)安備 11010802028692號