BOUTIQUEResources
Introduction
Oncolytic viruses, like herpes simplex virus (HSV) and vaccinia virus (VACV), are gaining traction in cancer therapy due to their selective replication in tumor cells, capacity to induce cell lysis, and ability to trigger anti-tumor immune responses. Similarly, pseudotyped lentiviral vectors (LVV), such as GalV and VSV-G, enhance gene therapy by enabling cell-type-specific transduction. This can can impact downstream purification strategies.
Traditional resin-based chromatography approaches face limitations in scalability, yield, and host cell protein (HCP) clearance. To address these bottlenecks, this study investigates a novel nanofiber-based adsorbent as an alternative purification strategy. This innovative platform offers promising advantages in binding capacity, impurity clearance, and process throughput, potentially transforming the downstream processing of large viral vectors.
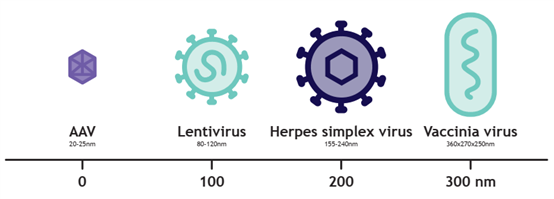
Larger viral vectors for gene therapy: LVV, HSV, and VACV
Aims of study
? Evaluate purification performance of a novel nanofiber adsorbent for LVV with different pseudotyped envelopes (VSV-G, 4070A, GalV) and oncolytic virus such as HSV
? Assess host cell driven proteins and dsDNA removal to determine impurity clearance capability
? Investigate scalability potential for industrial implementation of nanofiber-based purification
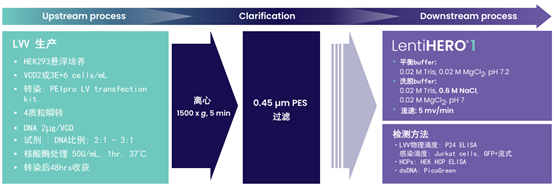
1. Pseudotyped LVV production and purification workflow
2. Higher binding capacity of VSV-G LVV particles using LentiHERO® nanofiber adsorbent
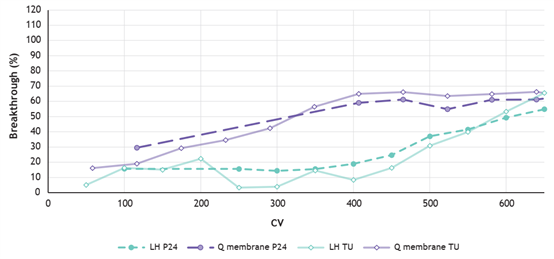
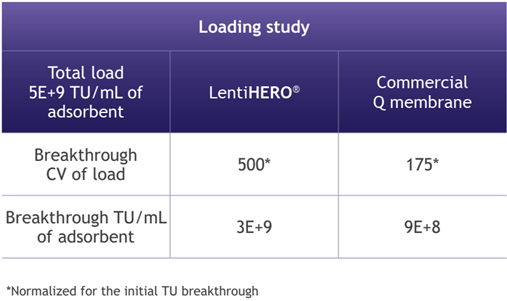
LentiHERO® processed large feed volumes before breakthrough of functional and physical LVV particles.
3. Maximizing the LVV recovery yield with high purity via optimized protocol
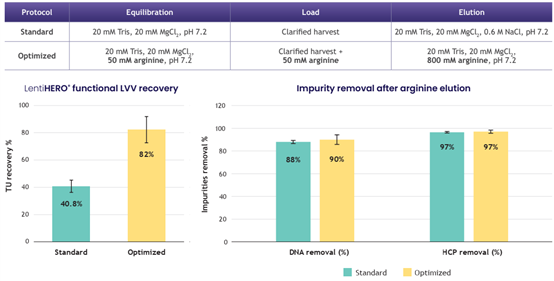
82% lentiviral recovery with yields > 2E+9 TU/mL of adsorbent and > 90% dsDNA/HCP clearance
4. Processing with LentiHERO® greatly reduces volumes for formulation at manufacturing scale
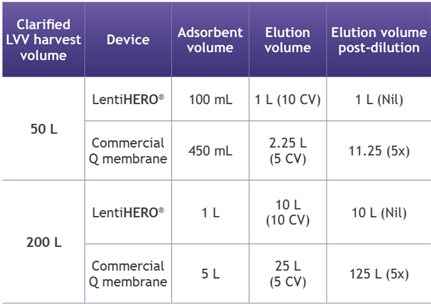
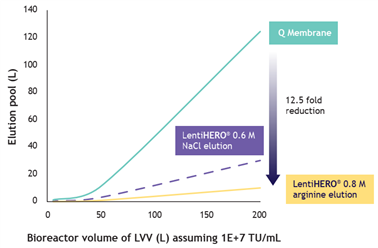
? Arginine elution eliminates the need for post-elution dilution, simplifying the process
? Low salt or arginine elution minimizes buffer consumption and reduces processing volume
5. Non-VSV-G pseudotyped LVV purification by LentiHERO®1
Lentiviruses can be pseudotyped with different envelope glycoproteins to enhance transduction efficiency and specific tropism. This impacts downstream purification strategies.
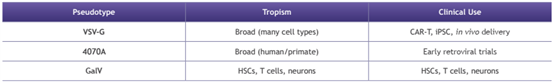

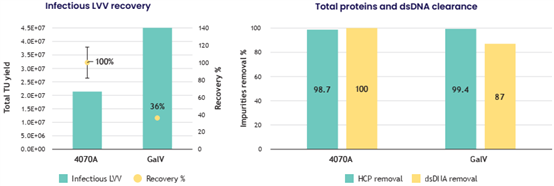
? Efficient purification of non-VSV-G lentiviral vectors (4070A: 100%, GalV: 36%) achieved
? Robust impurity reduction: >98% host cell proteins and >87% residual dsDNA without compromising vector functionality
6. Upstream-downstream workflow of oncolytic HSV-1

7. HSV purification profile on LentiHERO® using wt HSV-1(KOS)
Load volume 15 mL to a screening device measured 1.5E+10 IU/mL adsorbent.
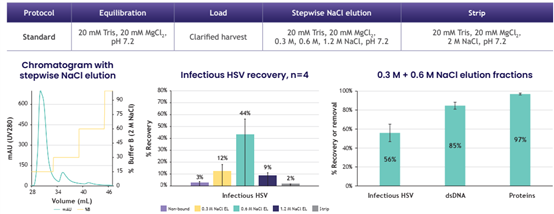
Infectious HSV recovery yield was 56%, 9.2E+9 IU/mL adsorbent.
Most infectious HSV eluted at 0.6 M NaCl.
8. HSV purification profile on LentiHERO® using pBACYAC-HSV-1(KOS)
Load volume 20 mL to a screening device measured on average 3E+8 IU/mL adsorbent.
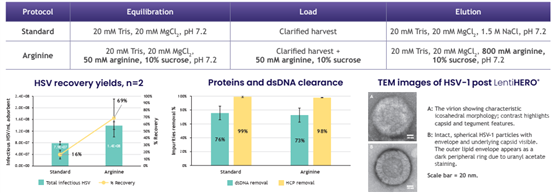
Infectious HSV recovery achieved 69%, ~1.4E+8 IU/mL adsorbent by 0.8 M arginine elution with high impurity clearance.
Conclusions
? LentiHERO® weak AEX nanofiber effectively purifies a range of viral vectors, including:
- Pseudotyped lentiviruses (4070A, VSV-G, GalV)
- Oncolytic virus HSV-1
? High recovery yields achieved:
- 4070A: 100%, VSV-G: 82%, GalV: 36%, HSV-1: 69%
? Superior host cell proteins clearance: >97% host cell protein removal
? Scalable and versatile platform suitable for industrial applications
? Potential expansion to other viral vectors like Ad5, Vaccinia, and VLPs
? Supports processing intensification for gene therapy and oncolytic virus manufacturing

Product Information
|
Product No. |
Prodcut Name |
Package |
|
NL100100 |
Nereus LentiHERO |
2 unit pack |
|
LH0001001A |
LentiHERO® 1 |
1 mL |
|
LH0010001B |
LentiHERO® 10 |
10 mL |
|
LH0010001C |
LentiHERO® 100 |
100 mL |
If you are interested in the above products, please click here to fill in the information. Astrea Bioseparations technical experts will provide you with personalized optimization solutions and effectively help you solve the difficult problems in the purification process.

Astrea Bioseparations was spun off from the University of Cambridge in 1987 and has over 30 years of experience in the research and development and production of chromatography media. It is a world-class supplier of chromatography media products and services. Currently, more than 21 production processes using Astrea's products have been approved by the FDA and EMA. Astrea Bioseparations has three R&D and production bases worldwide, focusing on providing industry-leading chromatography media and technical services for the fields of biologics and CGT. The new chromatography technology based on nanofibers launched by Astrea Bioseparations has solved the problems of low loading capacity and time-consuming processes of traditional biological separation tools, achieving a faster, more environmentally friendly and cost-effective purification process, fully meeting the needs of today's biopharmaceutical innovation.

XMJ Scientific is the agent of Astrea Bioseparations in China, providing users with comprehensive technical support and after-sales service. Welcome to call XMJ's customer service hotline at 400-050-4006 or visit the website www.mb020.com.cn for more information at any time.


.png) 京公網(wǎng)安備 11010802028692號(hào)
京公網(wǎng)安備 11010802028692號(hào)